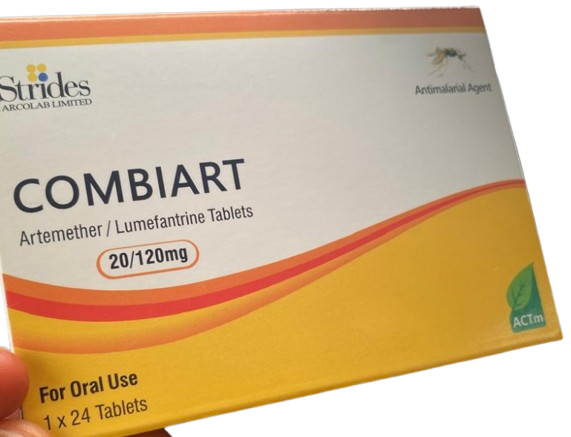
The National Agency for Food and Drug Administration and Control (NAFDAC) has issued a public alert regarding the circulation of counterfeit Combiart Dispersible Tablet 20/120mg, a malaria treatment, in Nigeria. The agency warned that the fake product, which is manufactured by Strides Arcolab Limited, an Indian-based company, poses significant health risks.
In a statement posted on its X (formerly Twitter) handle on Thursday, NAFDAC announced that it had begun a nationwide mop-up of the counterfeit product following its discovery in the Federal Capital Territory (FCT) and Rivers State during surveillance activities conducted by the agency’s Post Marketing Surveillance Directorate.
Laboratory Analysis Finds No Active Ingredients
Laboratory tests on the counterfeit tablets revealed that the product contained zero active pharmaceutical ingredients (APIs). Furthermore, the packaging of the counterfeit tablets bore two different date markings. NAFDAC confirmed that the product’s registration number was incorrect and did not correspond to the product, while the product license had expired.
The counterfeit Combiart tablets are part of a combination therapy used to treat malaria, a disease transmitted by mosquitoes. The genuine product contains Artemether and Lumefantrine, both of which are antimalarial drugs. However, NAFDAC emphasized that the counterfeit version is ineffective and does not provide the necessary treatment for malaria.
Health Risks of Counterfeit Medicines
NAFDAC warned that counterfeit or falsified medicines endanger public health because they do not meet regulatory standards. Such products fail to ensure the safety, quality, and efficacy required for effective treatment, potentially leading to serious health complications, including death.
The agency stressed that the use of substandard medicines like the counterfeit Combiart tablets may not only fail to treat the condition but can also worsen the patient’s health condition.
Product Details and Batch Information
The counterfeit Combiart tablets bear the batch number 7225119 and NAFDAC registration number A11-0299, which are incorrect. The manufacturing dates listed on the packaging are June 2023 and February 2023, with expiry dates of May 2026 and June 2026, respectively. The manufacturer is identified as Strides Arcolab Limited, located at 36/7, Suragajakkanahalli, Indlavadi Cross, Anekal Taluk, Bangalore-562 106, India.
NAFDAC Action and Public Advisory
NAFDAC has instructed all zonal directors and state coordinators to intensify surveillance and conduct a thorough mop-up operation to remove counterfeit products from the market. The agency urged importers, distributors, retailers, healthcare professionals, and caregivers to be vigilant when sourcing and distributing medical products.
Healthcare professionals and consumers are advised to report any suspected counterfeit medicines or medical devices to NAFDAC’s offices or via the agency’s official contact channels:
- NAFDAC Hotline: 0800-162-3322
- Email: sf.alert@nafdac.gov.ng
NAFDAC also encouraged reporting of any adverse events or side effects related to medicinal products through the E-reporting platforms on the NAFDAC website or via the Med-Safety application available for download on Android and iOS stores.